

Clinical trials in cosmetic dermatology have some intrinsic limitations. Some of the prerequisites of an RCT such as blinding, allocation concealment and even randomization is difficult to achieve in cosmetic dermatology. Hence some of the well known scales for the methodological assessment of clinical trials such as Jadad 1 and CONSORT 2 have limited relevance in clinical trial. Flawed evaluation of clinical trial quality allows flawed trials to thrive leading to clever new ways to distort trial results toward a favoured outcome.
I have tried to enumerate well known biases in cosmetic dermatology and to put these ideas together as a framework for evaluating clinical trials in cosmetic dermatology. I hope to further refine this with the help of Dermatologists Sans Borders, by first commenting on the validity, relevance and ease of assessment of the following biases. Subsequently we will attempt to collect data through DermSB for testing the inter and intra rater reliability of this tool. I have given it the acronym TASCDerm. Below are the assessment categories with score in brackets:
| Criteria (Maximum score) | Explanation | Score |
|---|---|---|
| Randomization (2) | 0 if no randomization performed or not mentioned, 1 if partial or inadequate, 2 if adequate. | |
| Placebo (2) | 0 if no placebo, 1 if inert placebo, 2 if compared to gold standard. | |
| Blinding (2) | 0 No blinding, 1 Single blinded, 2 Double | |
| Objective measurement (1) | Example: 1 if Colorimeter is used for melasma and 0 if MASI is used. | |
| Sample size more than 21 (1) | Effect size of 0.5, alpha 0.05 and power 80 | |
| Sample selection and allocation concealment (1) | 0 if study is done on clinic staff or if person knows allocated group. 1 if appropriate. | |
| Economical (1) | 1 if Intervention cheaper than gold standard. | |
| Conflict of interest (1) | 0 if present, 1 if absent | |
| Withdrawal / Dropouts (1) | 0 if more than 1 in 5 (20%) or not mentioned, 1 if not | |
| Coherent endpoints & assessment times (1) | 0 if not mentioned or inappropriate, 1 if appropriate. | |
| Co-Interventions (1) | 0 if multiple active ingredients tested, 1 if single. | |
| Acceptable theory (1) | 0 if no rational theoretical basis, 1 if sound. | |
| Total (15) |
Interpretation is as follows:
- 13-15 – Excellent study with substantial evidence
- 10-12 – Good study. Evidence sufficient for practice.
- 7-9 – Average Study. Use the evidence with caution.
- 4-6 – Weak Study. Evidence not suitable for practice.
- 0-3 – Just an anecdotal claim.
* if any of the criteria is not mentioned in the article/report being assessed, it is not scored.
Please cite TASCDerm as below:
Printable PDF of TASCDerm can be downloaded here.
WP-Dermatology is a WordPress plugin for dermatology bloggers and it supports TASCDerm. WP-Dermatology is free and can be downloaded here. You can add TASCDerm to journal article review posts without any programming using this plugin.
Do you want to try rating an article? Try this one! (Full Article available) ANTIAGING photoprotective and brightening activity in biorevitalization: a new solution for aging skin. (This link will show my annotations. Just click on the highlighted areas of the text and you can see my comments.)
Please share to view my assessment of the above article. But before you see the score I have given, Please do your rating first with this form.
[sociallocker id=771]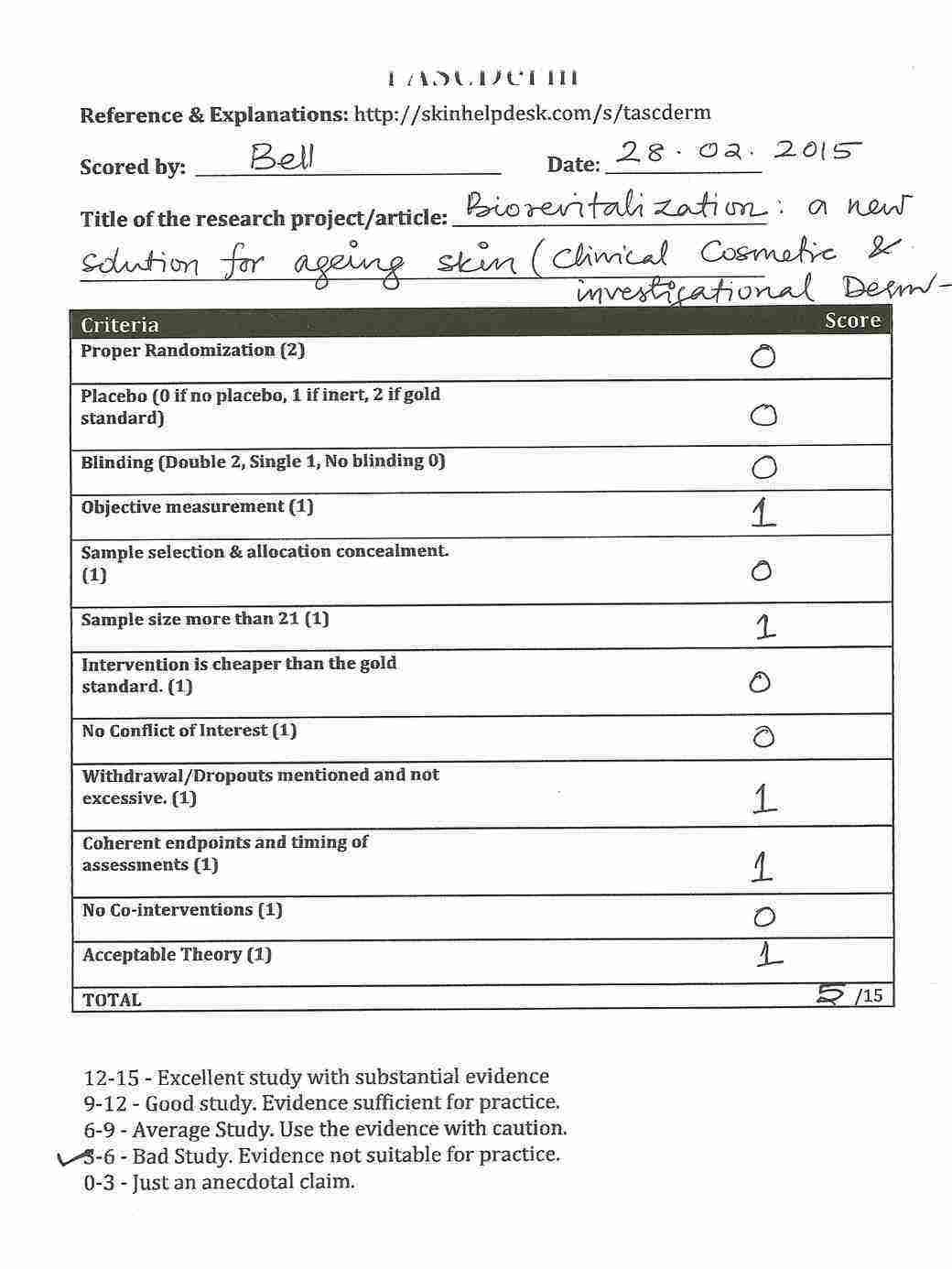
Please comment with the score you gave for the above article. Further detailed about TASCDerm can be found on the wiki.
Thanks Dr Sherina Laskar for suggestions.
This article was peer reviewed by Dr. Feroze Kaliyadan.
- Machine learning-based BOTOX API - April 11, 2023
- Skinmesh: Machine learning for facial analysis - November 10, 2020
- Free Dermatology EMR for Machine Learning and Artificial Intelligence - January 2, 2020
References:
- Jadad Alejandro R, MD, Dphil, Moore R Andrew, DPhil, Carroll Dawn, RGN, Jenkinson Crispin, DPhil, Reynolds D John M, DPhil, Gavaghan DavidJ, DPhil, Henry J, McQuay DM. Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary? Controlled Clinical Trials. 1996;17:1–12. ↩
- Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, Gøtzsche PC, Lang T. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134(8):663–694. ↩
1. The 15-point scoring system does not look complicated even to me (with my aforementioned handicaps).
2. A gold standard may not exist in some treatment scenarios, so wouldn’t the score be down by one in such cases?
3. Ditto with objective measurement
4. How do you double-blind a procedure involving, say, lasers?
5. Need to define ‘excessive drop-outs’
why do we need this. and what good will it do to me as a practitioner? May be at an institution level it may be feasible. I am looking forward to an economically rewarding solution.
This is not really a viable tool in practice keeping in mind the time constraints & the number of drop outs.
Like Dr Sherina rightly pointed out, several Cosmetic procedures can not be double-blinded.
In my set up , the main constraint would be getting the patients to turn up on the right day at the right time etc-
Pitfalls in clinical trials reveal need for well tolerated, more effective depigmenting agents. Only 30% of published trials were double-blind, 27% used a placebo and 80% used subjective measurements for their results.
Read More: http://informahealthcare.com/doi/abs/10.3109/09546634.2014.998609
For me TASCDerm is a great tool to bring more evidence in aesthetic dermatology and cosmetic market. Without extra rigor aesthetic dermatology procedures will remain under penetrated and a large part of patient / consumer will remain skeptical. Now we need to validate the robustness of this trial assessment scale